______________ Have Properties Of Both Metals And Non Metals.
trychec
Nov 06, 2025 · 9 min read
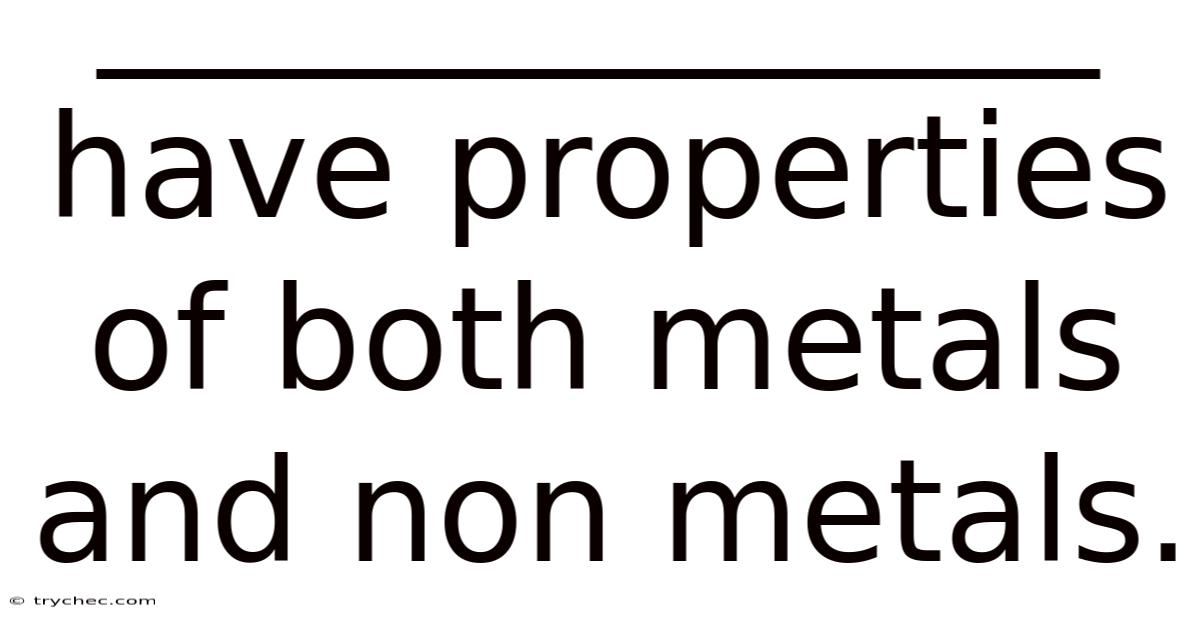
Table of Contents
Metalloids, also known as semi-metals, occupy a fascinating middle ground in the periodic table, possessing properties that blur the lines between metals and non-metals. These elements, which typically include boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), and tellurium (Te), exhibit a unique combination of characteristics that make them indispensable in various technological applications. Understanding the behavior of metalloids requires exploring their atomic structure, electronic configurations, and how these factors influence their physical and chemical properties.
Introduction to Metalloids
Metalloids are elements that straddle the boundary between metals and non-metals on the periodic table. This positioning gives them an intermediate set of properties, allowing them to behave as conductors or insulators under different conditions. This behavior is why they are often referred to as semi-metals.
The properties of metalloids are heavily influenced by their electronic structure. These elements tend to have partially filled electron shells, leading to their semiconducting abilities. The ability to fine-tune their electrical conductivity through doping makes them vital in semiconductor devices.
Key Properties of Metalloids
Metalloids possess a mix of metallic and non-metallic characteristics, making them unique. Key properties include:
- Semiconductivity: This is perhaps the most defining characteristic of metalloids. They conduct electricity better than insulators but not as well as metals. This intermediate conductivity can be altered by adding impurities, a process known as doping.
- Appearance: Metalloids often have a metallic luster but are brittle, a non-metallic trait. Their appearance can be deceptive, as their physical behavior does not always align with their looks.
- Chemical Behavior: Chemically, metalloids can react with both metals and non-metals, and their oxides are typically amphoteric, meaning they can act as both acids and bases.
Detailed Look at Specific Metalloids
Each metalloid has unique properties that make it suitable for specific applications. Here's a closer look at some of the most important metalloids:
-
Boron (B)
- Properties: Boron is a hard, brittle, black metalloid. It has a high melting point and is a poor conductor of electricity at room temperature.
- Uses: Boron compounds are used in the production of heat-resistant glass (borosilicate glass), detergents, and as a neutron absorber in nuclear reactors. Boron fibers are used in high-strength, lightweight composite materials.
-
Silicon (Si)
- Properties: Silicon is the most well-known metalloid, with a grayish-black metallic appearance. It is a semiconductor and is crucial in the electronics industry.
- Uses: Silicon is the primary material used in the manufacture of integrated circuits, transistors, and solar cells. It is also used in the production of silicones, which are polymers used in lubricants, adhesives, and sealants.
-
Germanium (Ge)
- Properties: Germanium is a hard, grayish-white metalloid that is also a semiconductor.
- Uses: Like silicon, germanium is used in transistors and other semiconductor devices. It is also used in infrared optics and as a catalyst in polymerization reactions.
-
Arsenic (As)
- Properties: Arsenic is a brittle, steel-gray metalloid that can exist in several allotropic forms. It is toxic and its compounds are poisonous.
- Uses: Historically, arsenic compounds were used as pesticides and wood preservatives. Today, arsenic is used in semiconductor materials and in certain alloys to improve their properties.
-
Antimony (Sb)
- Properties: Antimony is a silvery-white metalloid that is relatively stable in air and water.
- Uses: Antimony is used in alloys to increase their hardness and strength. It is also used in flame retardants, batteries, and as a component in semiconductors.
-
Tellurium (Te)
- Properties: Tellurium is a silvery-white metalloid that is brittle and easily pulverized.
- Uses: Tellurium is used in alloys to improve their machinability. It is also used in solar cells and as a vulcanizing agent in rubber production.
The Science Behind Metalloid Properties
The dual nature of metalloids arises from their electronic configurations and atomic structures. Here's a deeper look into the scientific principles that govern their behavior:
- Electronic Configuration: Metalloids typically have electron configurations that are intermediate between those of metals and non-metals. This results in moderate electronegativity values, allowing them to form both covalent and ionic bonds.
- Semiconductivity Explained: The semiconducting properties of metalloids are due to their energy band structure. In a semiconductor, there is a small energy gap between the valence band (where electrons reside) and the conduction band (where electrons can move freely). This gap allows electrons to jump to the conduction band with a small amount of energy, enabling electrical conductivity.
- Doping: Doping involves adding impurities to a semiconductor to increase its conductivity. Adding elements with more valence electrons (n-type doping) or fewer valence electrons (p-type doping) creates an excess of electrons or holes, respectively, enhancing conductivity.
Applications of Metalloids in Modern Technology
Metalloids play a crucial role in modern technology, particularly in electronics, materials science, and renewable energy. Their unique properties make them indispensable in various applications:
- Electronics Industry: Metalloids, especially silicon and germanium, are the backbone of the electronics industry. They are used to manufacture transistors, diodes, and integrated circuits, which are the building blocks of computers, smartphones, and other electronic devices.
- Solar Cells: Silicon and tellurium are used in the production of solar cells, which convert sunlight into electricity. These materials are chosen for their ability to absorb sunlight and efficiently generate electrical current.
- Alloys and Materials Science: Metalloids are used to modify the properties of alloys and composite materials. For example, adding antimony to lead increases its hardness, while boron fibers are used to create lightweight, high-strength composites for aerospace applications.
- Medicine: Some metalloid compounds have medicinal applications. For example, arsenic compounds have been used in the treatment of certain types of cancer, although their toxicity requires careful handling and precise dosages.
Advantages and Disadvantages of Using Metalloids
Like any class of materials, metalloids have their own set of advantages and disadvantages that must be considered when choosing them for specific applications:
Advantages:
- Tunable Conductivity: The ability to control the conductivity of metalloids through doping makes them ideal for semiconductor devices.
- Versatility: Metalloids can form a wide range of compounds with diverse properties, making them useful in various applications.
- Unique Properties: The combination of metallic and non-metallic properties allows metalloids to perform functions that neither metals nor non-metals can achieve alone.
Disadvantages:
- Toxicity: Some metalloids, such as arsenic, are highly toxic, requiring careful handling and disposal.
- Brittleness: Many metalloids are brittle, limiting their use in structural applications where ductility is required.
- Complexity in Processing: The processing and purification of metalloids can be complex and expensive, adding to the cost of manufacturing devices that use them.
The Future of Metalloid Research
Research into metalloids continues to evolve, with ongoing efforts to discover new applications and improve existing technologies. Some promising areas of research include:
- Nanomaterials: Metalloid nanomaterials, such as silicon nanowires and boron nitride nanotubes, exhibit unique properties that could lead to new electronic devices and sensors.
- Thermoelectric Materials: Metalloid compounds are being explored as thermoelectric materials, which can convert heat into electricity and vice versa. These materials could be used in waste heat recovery and solid-state cooling applications.
- Advanced Alloys: Researchers are investigating new alloys containing metalloids to create materials with enhanced strength, corrosion resistance, and other desirable properties.
Metalloids in Everyday Life
Although often unseen, metalloids play a crucial role in our daily lives. From the smartphones we use to communicate to the solar panels that generate clean energy, metalloids are essential components of modern technology. Here are a few examples of how metalloids impact our everyday lives:
- Smartphones and Computers: Silicon is the foundation of the microchips that power our smartphones, computers, and other electronic devices. Without silicon, these devices would not be possible.
- Renewable Energy: Solar panels, which convert sunlight into electricity, rely on silicon and tellurium to capture and convert solar energy.
- Automobiles: Metalloids are used in various components of automobiles, including semiconductors in engine control systems and alloys in structural parts.
- Construction: Boron compounds are used in the production of borosilicate glass, which is used in laboratory glassware, cookware, and windows due to its high heat resistance and low thermal expansion.
Conclusion
Metalloids represent a unique and fascinating class of elements, possessing properties that bridge the gap between metals and non-metals. Their semiconducting abilities, combined with their diverse chemical behavior, make them indispensable in modern technology. From the microchips that power our electronics to the solar panels that generate clean energy, metalloids play a crucial role in our daily lives. As research continues to uncover new applications and improve existing technologies, the importance of metalloids is only set to grow in the future. Understanding their properties and behavior is essential for advancing technology and creating a more sustainable world.
FAQ About Metalloids
-
What are metalloids?
- Metalloids, also known as semi-metals, are elements that have properties of both metals and non-metals. They are typically defined as boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), and tellurium (Te).
-
What makes metalloids unique?
- Metalloids are unique because they exhibit an intermediate set of properties between metals and non-metals. Their semiconductivity, ability to form both covalent and ionic bonds, and amphoteric oxides distinguish them from other elements.
-
What is semiconductivity?
- Semiconductivity is the ability of a material to conduct electricity better than an insulator but not as well as a metal. This property is crucial for electronic devices such as transistors and diodes.
-
How is the conductivity of metalloids controlled?
- The conductivity of metalloids can be controlled through a process called doping. Doping involves adding impurities to the metalloid to increase its conductivity by creating an excess of electrons (n-type doping) or holes (p-type doping).
-
What are some common applications of metalloids?
- Metalloids are used in a wide range of applications, including:
- Electronics: Silicon and germanium in transistors, diodes, and integrated circuits.
- Solar Cells: Silicon and tellurium in solar panels.
- Alloys: Antimony to increase the hardness of lead, boron fibers in high-strength composites.
- Medicine: Arsenic compounds in certain cancer treatments.
- Metalloids are used in a wide range of applications, including:
-
Are metalloids safe to handle?
- Some metalloids, such as arsenic, are highly toxic and require careful handling and disposal. Others, like silicon, are relatively safe. It is essential to follow proper safety protocols when working with any metalloid.
-
What is the future of metalloid research?
- Future research on metalloids includes the development of nanomaterials, thermoelectric materials, and advanced alloys. These areas hold promise for new electronic devices, energy technologies, and materials with enhanced properties.
-
Why are metalloids also called semi-metals?
- Metalloids are also called semi-metals because they possess properties that are intermediate between those of metals and non-metals. This term reflects their dual nature and unique behavior.
-
Can metalloids be found in nature?
- Yes, metalloids can be found in nature. They occur in various minerals and compounds. For example, silicon is one of the most abundant elements in the Earth's crust, while arsenic is found in minerals such as arsenopyrite.
-
What is the role of metalloids in environmental science?
- Metalloids play a role in environmental science due to their presence in soil, water, and air. Some metalloids, such as arsenic, can be environmental pollutants, while others, like silicon, are essential for plant growth and ecosystem health.
Latest Posts
Latest Posts
-
Terrorism Is The Spontaneous Use Of Violence
Nov 06, 2025
-
List Three Artistic Tasks The Monks And Nuns Performed
Nov 06, 2025
-
Which Helps Enable An Oligopoly To Form Within A Market
Nov 06, 2025
-
Interventions Designed To Prevent Problem Behaviors
Nov 06, 2025
-
A New Recipe Is Introduced To The Foodservice Operation Servsafe
Nov 06, 2025
Related Post
Thank you for visiting our website which covers about ______________ Have Properties Of Both Metals And Non Metals. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.